Theme: Essential innovation in pharma biosimilars.
Pharma Biosimilars 2019
Pharma Biosimilars 2019 invites all the participants from all over the world to attend “International Conference on Pharma Biosimilars and Pharmaceutical” (Pharma Biosimilars 2019) slated on February 28, March 01, 2019 Osaka, Japan which covers on all aspects of the pharmaceutical sciences, manufacturing, quality with strong emphasis on originality and scientific quality
Conference Series LLC LTD organizes 1000+ Conferences every year across USA, Europe & Asia with support from 1000 more scientific societies and publishes 1000+ Open access journals which contain over 70000 eminent personalities, reputed scientists as editorial board members, 1200 Symposium & Workshops and 5 million followers. Conference Series Ltd invites all the participants across the globe to attend the “International Conference on Pharma Biosimilars and Pharmaceutical"
Pharma Biosimilars 2019 conveys recent developments in Pharma drug marketing and production of Pharma drugs and contract manufacturing. A complete knowledge of a scientific discipline that described the effects of Biosimilars drug marketing and Biosimilars Pharma now explores the Scope of Biosimilars drug marketing in industry. Pharma Biosimilars 2019 provides detailed market, technology, and industry analyses to help readers quantify and qualify the market for prescription generic drugs. Important trends are identified and sales forecasts by product categories and major country markets these are based on industry sources and considered assessment of the regulatory environment, healthcare policies, demographics, and other factors that directly affect the generic drug market. The wider economic environment is also considered.
Why attend Pharma Biosimilars 2019:
Keynote presentation along with interactions to galvanize the scientific community.
Workshop and symposiums to reach the largest assemblage of participants from the Pharma/Biotech community.
A wide track of exhibitors to showcase the new and emerging technologies.
Platform to global investment community to connect with stakeholders in Pharma/Biotech sector.
Young Scientist/ Investigators Award geared towards best budding young research.
Links to the political marketing resources to expand your business and research network.
Triumph of Awards, Certificates recognizes your commitment to your profession to encourage the nascent research.
​Target Audience:
- Directors, CEO’s of Organizations
- Business Development Managers
- Chief Scientific Officers
- R&D Researchers from Biosimilar and Biologics Industries
- Professors, Associate Professors, Assistant Professors
- PhD Scholars
- Patent Attorneys
- Intellectual Property Attorneys
- Investment Analysts
- Association, Association presidents and professionals
- Noble laureates in Health Care and Medicine
- Bio instruments Professionals
- Bio-informatics Professionals
- Software development companies
- Research Institutes and members
- Supply Chain companies
- Manufacturing Companies
- CRO and DATA management Companies
- Training Institutes
- Business Entrepreneurs
Pharma Biosimilars 2019 has everything you need:
Open panel discussions: Providing an open forum with experts from academia and business to discuss on current challenges in Biosimilars & Biologics, where all attendees can interact with the panel followed by a Q&A session.
Speaker and poster presentations: Providing a platform to all academicians and industry professionals to share their research thoughts and findings through a speech or a poster presentation.
Editorial board meeting: Discussing on growth and development of open access Bioanalysis & Biomedicine International Journals and recruiting board members and reviewers who can support the journal.
Round table meetings: Providing a platform where industry professionals meet academic experts.
Over 50+ organizations and international pavilions will be exhibiting at the pharma Biosimilars 2019 conference. Exhibitors will include equipment manufacturers and suppliers, systems providers, finance and investment firms, R&D companies, project developers, trade associations, and government agencies.
In addition to the products and services you will have access to valuable content, including Keynote Presentations, Product Demonstrations and Educational Sessions from today’s industry leaders.
The Pharma Biosimilars 2019 has everything you need, all under one roof, saving you both time and money. It is the event you cannot afford to miss!
A two-day gathering that examines the future market patterns, creative business methodologies and open doors for development of moderate medications. Pharma pioneers want the substance and roundtable examinations yet remain for the systems administration and air. This meeting is formally the biggest key nonexclusive gathering in the business and will give members a thorough survey of business methodology for moderate meds.
Get your image before senior chiefs
Break into the lucrative Generic Medicines industry
Position your organization as an industry pioneer
Make deals leads and convey an arrival on venture
Connect with your objective market in the locale.
You will meet:
Board level and senior agents from nonspecific and biosimilar pharmaceutical organizations
Venture banks
Law offices
Track 1: Analytical Strategies of Biosimilar
Biologics and Biosimilar analysis make the one of the most important aspect on the development on biological products. The use of production technologies such as disposable and supply chain logistics can help companies such establish flexibility facility involved in biosimilar analytical methods.
Bioanalytical methods include in all global events of biosimilars for example: GMP protein analysis, pre-clinical and clinical program, Bioassay for comparability and potency testing.
Track 2: Emerging Biosimilars In Therapeutics
In the field of biologics, the new avenue has created towards clinicians because explorations have created for better disease management. In the treatment of ailments like psoriasis, rheumatic arthritis, certain cancer inflammation, bowel diseases etc. showed some good outcomes because of using biologics. Biosimilar insulins which are newly formed are likely to enter the insulin landscape as patents for major branded insulin products ready to expire in the next few years. The emerging biosimilar like filgrastim, peg filgrastim, recombinant blood products, therapeutic products vaccine biosimilar, anti-bodies growth hormones, biosimilar peptide, all these are used to sound knowledge on biosimilars development on drugs.
Track 3: Current Challenges in Developing Biosimilars
For the development of biological calls many changes may occur. Concepts of biologics with considerations at initial stages, clinical developments are essential at early. For the successful development of biologics follow on proper scientific and strategic approaches are need. Most probably, the drug safety factors and labelling requirements are the overcoming challenges and important in continue of late clinical steps. And now it is much required to develop a drug product in according to quality by design.
Track 4: Regulatory Approaches of Biosimilars
biosimilars is likewise called as nonspecific adaptation of biosimilars. The clinically idle compound is not withstanding with biosimilars but rather minor contrasts and profoundly like authorized item; additionally, there Presently a-days in pharmaceutical industry biosimilars is shaped has another buzz. Furthermore, these are no clinically important contrasts between the biologicals and the reference item as far as intensity, wellbeing and immaculateness. The worldview of customary generics to biosimilars, and different parts of Biosimilar endorsements. Also, these Biosimilars 2019 will give a phenomenal and overall chance to the researchers, accomplices and pharma pioneers from Biopharmaceutical and Biotechnology businesses to improve and to investigate the key market for Biosimilars and Biologics with an unmistakable photo of the administrative approach for biosimilars and biologics. The US biosimilars, European biosimilars, Canada biosimilars, UK biosimilars take a noteworthy part in cross the globe by 2019. Biosimilars development. And these biosimilar conferences will mainly focus on bio similar product development to deliver safe and successfully.
Track 5: Pharmacovigilance challenges in Biosimilars
Same proportion pharmacovigilance for biosimilars has comparatively more different from other products. Biosimilars 2019 session mainly look that have already been announced to include enhanced tracking and follow-up of post marketing surveillance issues, and on FDA initiative planned different changes in AERS, pilots of new developed post market strategies of drug-monitoring and ADR related problems. The guidelines of biosimilars for pharmacovigilance and pharma epidemiology are the points that shall be laid emphasis in the session In USA average annual income mostly spend on biologics comparing to generic drugs for example:16% on biologics and 3% on other drugs.
Track 6: BCS and IVIVC Based on Biosimilars
These Mainly discusses about different types of drugs and adsorption distribution metabolism excretion pathways. And This biosimilars includes, In vitro diffusion cells for dissolution testing in formulation development, The preclinical testing of ADME in INVITRO. The primary moto of this work was to suggest the biosimilars potential of biopharmaceutical classification system which are known to increase the dissolution. These insoluble drugs which is orally absorbed. These Aims at addressing all such challenges of the pharma formulation sector at biologics and biosimilars at 2019 conference. The solubility includes in biopharmaceutical classification.
Track 7: Globalization of Bio Similar
These tracks mainly deal with biosimilar meetings those who can attend by following biologics/protein/biosimilar product process science, New biosimilar development, portfolio Management, Reasearch&development, biosimilar market, business operations, scientific affairs. This track mainly discusses about Risk-sharing arrangement LATAM market for bio-similar adopting innovative mechanism for generic drugs impact on global bio similar market and cost and riskmanagement.
Track 8: Clinical Development of Biosimilars
Risk Management, quality affairs, Case studies, clinical models, Transgenic animals Targeted cell-line development, clinical biosimilar tracks are major diseases of clinical trials. The pkd/docx studies toxicological studies and Aspects of genotoxicity tests. And These tracks are designed especially for those who are having more knowledge on clinical studies and clinicians’ prospects for biosimilars. These guidelines on the above-mentioned topics are also to be thrown effect upon at this biosimilars conference
Track 9: Bioequivalence assessment
The active ingredient from the pharma product and its absorption into the systemic circulation is mainly focus on equivalence release. Bioequivalence assessment includes strategies of topical dosage forms and bio equivalence approaches for transdermal dosage form and assessment of respiratory dosage form.
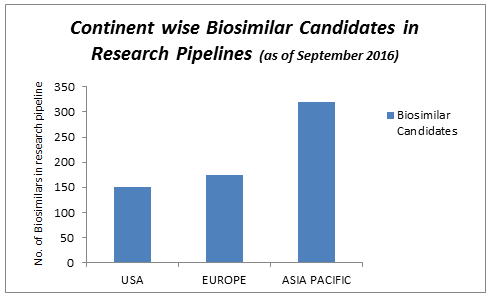
Track 10: Biosimilars Research Pipeline
The biological medical product is a biosimilar manufactured by a different company by the copy of an original product. The original biosimilar innovator products are officially approved biosimilar can be manufactured when the originally product patient expire. This session shall be highly useful for the biosimilar industry researchers to update themselves on the latest research updates from all over the world. Not only these sessions and also there are many ways to find the exact place for all the biosimilar exhibitions associated with present generation of biosimilars and biologics.
Track 11: Intellectual Property Rights
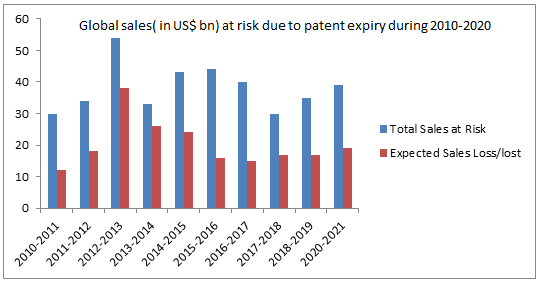
That any pharma product has successfully pass through the regulatory affairs scanners before launched in the market need less to mention. Biosimilars and biologics are no exceptions. The companies developing these biosimilars and biologics apart from regulatory aspects tend to enjoy monopoly over their product. Intellectual Property Rights (IPR) and patents are ultimate tools to the biosimilar manufacturers for safeguarding their interests. 2016 was a record year for development of Biosimilars & biologics in US. In this year 16 IPR petition was filed. So, expected scenario of 2017 would be a boom in Biosimilar and biologics field
Track 12: Brexit Effect on Biosimilar
The negative tendency of biosimilars Brexit impact. The hindrance towards the cost cutting approach taken by the NHS is a major setback towards approval and launch of biosimilars in market. With Britain being among foremost clinical preliminary focuses is claimed to see a diminishing in the eagerness of the makers and specialists to do any further preliminaries in Britain. Additionally, Brexit will cause the primary rationale of British Biosimilars Association (BBA) to fall back-which went for expanding the utilization of Biosimilars.
Track 13: Drug Delivery and Development
Medication Delivery Companies and Market session is starting to change for little, large, and medium scale pharmaceutical, Co biopharmaceutical Manufacturing and Industries, bland medications organizations, contract sedate conveyance organizations which can show from improvement to assembling. Tending to these dangers is an extraordinary test, because of the many-sided quality of the Clinical bio therapeutics themselves.
Track 14: Current Agency Expectations for Approval for Biosimilars
Biosimilars are a generally new subset of biopharmaceuticals, with the biotechnology business at last developing to such an extent that off-patent nonexclusive compose items progressively will enter real markets. Up until now, more than 20 biosimilars for a set number of reference items have been affirmed in real markets, principally the European Union. Just two items have been formally endorsed as biosimilars in the United States. The parent field of biopharmaceuticals itself keeps on displaying a poor supporting framework of data assets. Those biopharmaceutical and biosimilar data assets that do exist for the most part are restricted in number, assorted variety, and advancement
Track 15: Biological Medicine:
Organic Medicine works with the science of the body and its regular recuperating capacities and in addition the otherworldly, passionate and physical parts of malady. Sickness implies that the body's direction isn't working legitimately and should be brought once again into its normal powerful state where the invulnerable framework is in full control. It in this manner searches for main drivers for the showing side effects of sickness the hidden elements making a man give a specific disease. These underlying drivers may comprise of a few elements which have developed after some time and can incorporate; consume less calories, sustenance sensitivities, intestinal unsettling influences, family history, push, ecological elements, substantial metals, dental issues, hyperacidity, injury, introduction to microscopic organisms or infections or electromagnetic aggravation
Asia-Pacific Biosimilars Market was worth USD 1.26 million in 2018 and estimated to be growing at a CAGR of 31.60%, to reach USD 4.99 million by 2023 Biosimilars are emerging as one of the most important sector in the healthcare industry. With increasing healthcare costs, biosimilars are being looked upon as an affordable treatment option. Growing economies are observing comprehensive growth in biosimilars industry from demanding clinical needs of therapeutics. Rise in GDP and healthcare expenditures, and the demand for cost effective therapeutics solution has resulted in the growth of this market.
Biosimilars of Complex mAb structure are expected to grow to a cumulative market size of ¥300b in the next three years in Japan. It will open doors for the first oncology biosimilar mAb in Japan after Remicade BS launch in 2014, while over the next 7 years it is anticipated to be worth a cumulative ¥600b.
In the last five years, most of the companies have some alliance in place for biosimilars, with most of the Japanese companies undertaking pacts with South Korean companies to ride on the back of biosimilar mAb expertise. There is a trend of seeking out product specific alliances by most of the JP companies active in the BS space and to go step by step on these high risk/high return opportunities. Overall, in Japan's market, each opportunity has a different competitive landscape for itself, and some companies are looking for niche opportunities in biosimilar space as per their specialty therapy area- like ophthalmology BS (Lucentis), Enzyme therapy BS (JCR).
Asia-Pacific Biosimilars Market was worth USD 1.26 million in 2018 and estimated to be growing at a CAGR of 31.60%, to reach USD 4.99 million by 2023 Biosimilars are emerging as one of the most important sector in the healthcare industry. With increasing healthcare costs, biosimilars are being looked upon as an affordable treatment option. Growing economies are observing comprehensive growth in biosimilars industry from demanding clinical needs of therapeutics. Rise in GDP and healthcare expenditures, and the demand for cost effective therapeutics solution has resulted in the growth of this market.
Biosimilars of Complex mAb structure are expected to grow to a cumulative market size of ¥300b in the next three years in Japan. It will open doors for the first oncology biosimilar mAb in Japan after Remicade BS launch in 2014, while over the next 7 years it is anticipated to be worth a cumulative ¥600b.
Asia Pacific Biotech Congress 2017
Conference series hosted 15th Asia Pacific Biotechnology Congress during July 20-22, 2017 Melbourne Australia, based on the theme “Novel Innovations and Strategies for Sustainable Health”. Active participation and generous response were received from the Organizing Committee Members, scientists, researchers, as well as experts from Non-government organizations, and students from diverse groups who made this conference as one of the most successful and productive events in 2017 from Conference series.The conference was marked with several workshops, multiple sessions, Keynote presentations, panel discussions and Poster sessions. We received active participation from scientists, young and brilliant researchers, business delegates and talented student communities representing more than 35 countries, who have driven this event into the path of success.
The conference was initiated with a warm welcome note by Honorable guests and the Keynote forum. The proceedings went through interactive sessions and panel discussions headed by honorable Moderator Petr Maly BIOCEV research Organization center. Czech Republic for the conference.The conference proceedings were carried out through various Scientific-sessions and plenary lectures, of which the following Speakers were highlighted as Keynote speakers:
Genetic engineering of tobacco plants by expressing arsenic responsive genes of Lysin bacillus spherical and Arabidopsis thaliana for removal of arsenics from the contaminated lands: Abul Mandal, University of Skived, Sweden.Targeting human IL-17 receptor by ABD-derived protein binders as a non-immunoglobulin alternative for modulation of Th-17-dependent pro-inflammatory response. Petr Maly, BIOCEV Research Center, Czech Republic
Conference series has taken the privilege of felicitating Asia Pacific Biotechnology Congress Organizing Committee, Keynote Speakers who supported for the success of this event. Conference series, on behalf of the Organizing Committee congratulates the Best Poster awardees for their outstanding performance in the field of Biotechnology and appreciates all the participants who put their efforts in poster presentations and sincerely wishes them success in future endeavors.
Poster Judging was done by: Petr Maly BIOCEV research Organization center. Czech Republic, Best Poster Award was received by: Cindy Babura, Vaal University of Technology.We are also obliged to various delegate experts, company representatives and other eminent personalities who supported the conference by facilitating active discussion forums. We sincerely thank the Organizing Committee Members for their gracious presence, support, and assistance towards the success of 15th Asia Pacific Biotechnology Congress.
Conference Highlights
- Analytical Strategies of Biosimilar
- Emerging Biosimilars In Therapeutics
- Current Challenges in Developing Biosimilars
- Regulatory Approaches of Biosimilars
- Pharmacovigilance challenges in Biosimilars
- BCS and IVIVC Based on Biosimilars
- Globalization of Bio Similar
- Clinical Development of Biosimilars
- Bioequivalence assessment
- Biosimilars Research Pipeline
- Intellectual Property Rights
- Brexit Effect on Biosimilar
- Drug Delivery and Development
- Current Agency Expectations for Approval for Biosimilars
- Biological Medicine
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | Feb 28-March 01, 2019 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | Day 1 | ||
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
- Journal of Bioanalysis & Biomedicine
- Journal of Bioequivalence & Bioavailability
- Journal of Pharmaceutical Sciences & Emerging Drugs
Abstracts will be provided with Digital Object Identifier by




